
Sodium Bohr model
The main points of Bohr's model are as follows: 1. Electrons move in fixed orbits and not anywhere in between. 2. Each orbit has a fixed size and energy. The energy of the orbit is related to.
Sodium (Na) AMERICAN ELEMENTS
Physical & Theoretical Chemistry Supplemental Modules (Physical and Theoretical Chemistry) Electronic Structure of Atoms and Molecules
Sodium (Na) AMERICAN ELEMENTS
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV
.PNG)
Bohr Models and Lewis Dot Diagrams Presentation Chemistry
The energy for the first energy level is equal to negative 13.6. E two is equal to negative 3.4, and E three is equal to negative 1.51 electron volts. So energy is quantized using the Bohr models, you can't have a value of energy in between those energies.

Sodium Facts & Bohr Model YouTube
It is the amount of energy that an electron gains when subjected to a potential of 1 volt; 1 eV = 1.602 × 10 -19 J. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n = 5 to the orbit with n = 2. Show your calculations.
Bohr Model of Sodium Science ShowMe
With sodium, however, we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum, at about 589 nm. Figure \(\PageIndex{1}\): The Emission of Light by Hydrogen Atoms. (a) A sample of excited hydrogen atoms emits a characteristic red light.. Bohr's model required only one assumption:.

Draw a sketch of Bohr's model of a sodium atom. Brainly.in
or. 1 λ = k hc( 1 n2 1 − 1 n2 2) The lowest few energy levels are shown in Figure 3.2.1. One of the fundamental laws of physics is that matter is most stable with the lowest possible energy. Thus, the electron in a hydrogen atom usually moves in the n = 1 orbit, the orbit in which it has the lowest energy.

Bohr Model Of Sodium Best Diagram Collection
Niels Bohr adapted Ernest Rutherford's nuclear model. Bohr did calculations that led him to suggest that electrons orbit the nucleus in shells. The shells are at certain distances from the nucleus.

Atom illustration, Bohr model Sodium Atom Chemistry Rutherford model
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.
Bohr Model Of A Phosphorus Atom Electron Configuration Of Sodium
atom On the Web: Space.com - The Bohr model: The famous but flawed depiction of an atom (Jan. 08, 2024) See all related content → Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.

Bohr Diagram Of Sodium Atom model, Atom diagram, Atom model project
Bohr's model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits. Bohr found that an electron located away from the nucleus has more energy, and the electron which is closer to nucleus has less energy Postulates of Bohr's Model of an Atom
Sodium Facts & Bohr Model YouTube
Steps to draw the Bohr Model of Sodium atom 1. Find the number of protons, electrons, and neutrons in the Sodium atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom

Sodium Bohr Model — Diagram, Steps To Draw Techiescientist
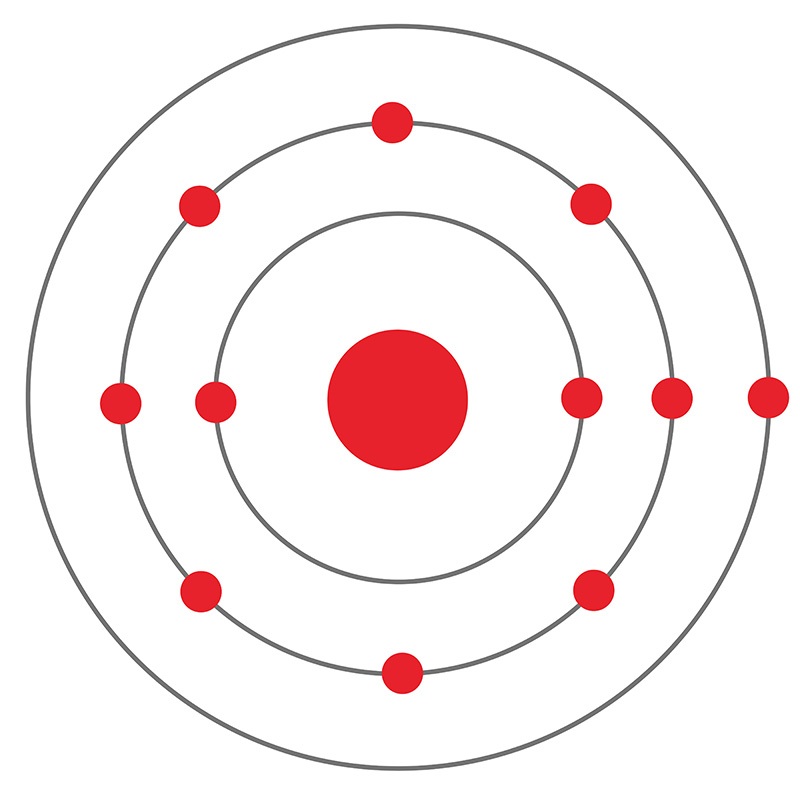
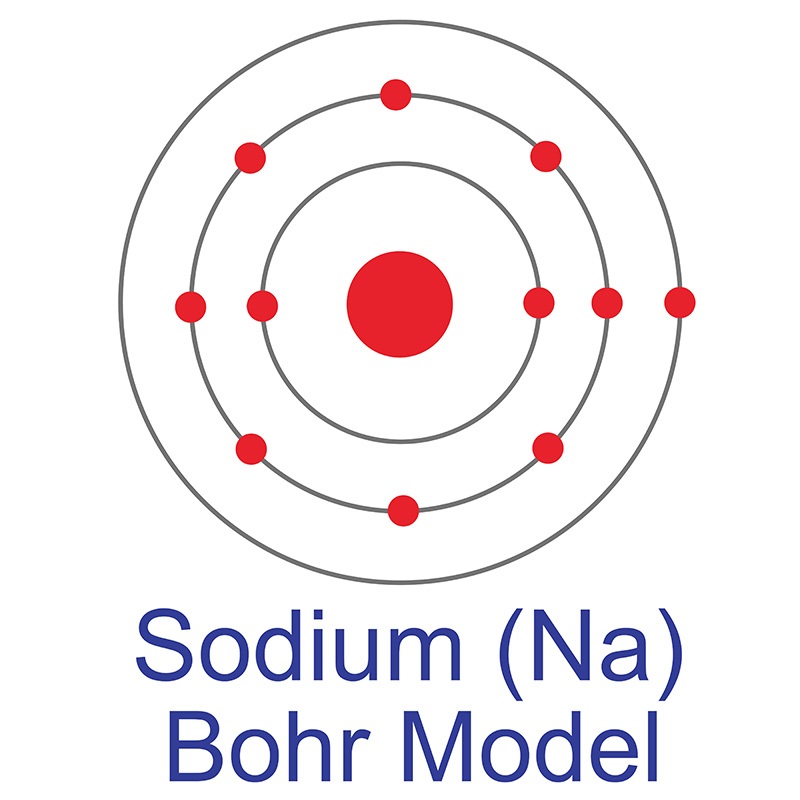
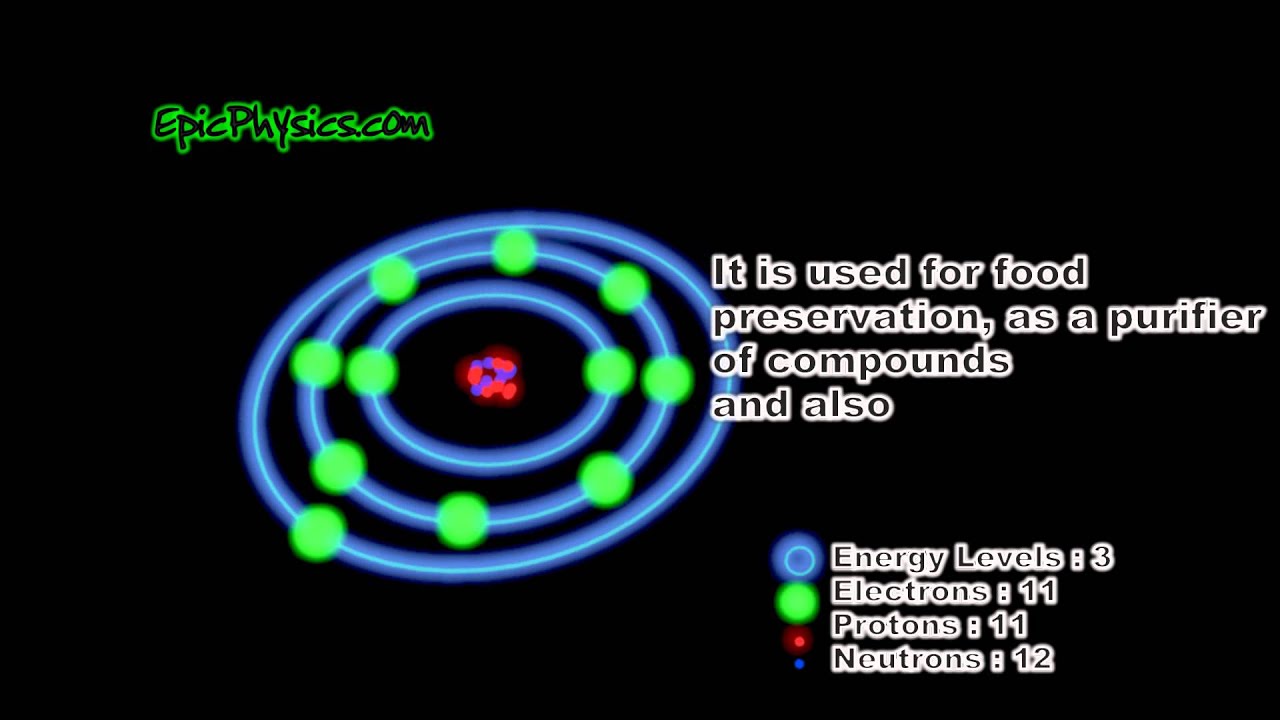
The Bohr model of sodium contains a nucleus having 11 protons and 12 neutrons in the center, and around this nucleus, there are three electron shells containing 11 electrons. Atomic Structure of the Sodium Atom (Na) Watch on Contents Steps #1 Write protons, neutrons, and electrons of sodium atom #2 Draw nucleus of sodium atom

Bohr Diagram For Calcium Wiring Diagram
Contents show Bohr Model of Sodium The Bohr-Rutherford model was given in 1913 after incorporating the findings of Niel Bohr in the already given Bohr model. The Bohr model is a representation of the atomic structure along with all the atomic particles in pictorial form.

3D Bohr Model of a Sodium26 Isotope Atom YouTube
The Bohr Model of Sodium. Let's look at the Bohr model of sodium, which has 11 electrons. Image Courtesy of Wikimedia. The atomic number of sodium is 11, which indicates that there are both 11 protons and electrons. This is why there are 11 electrons represented in the above diagram.

Atom Sodium Model Stock Illustration Download Image Now iStock
Creating a sodium Bohr diagram is a way to visually represent the arrangement of electrons in a sodium atom based on the Bohr model. The Bohr model suggests that electrons occupy specific energy levels or shells around the nucleus of an atom. Each shell can hold a certain number of electrons, and the diagram helps to show the distribution of.